DHEA, Nano CBD, The Healthy Skeptics
Announcing: Altea NANO CBD

Announcing: Altea NANO CBD
In this newsletter:
- The Endocannabinoid System (ECS)
- Confusion reigns
III How is Altea Nano-CBD different / better?
- Questions we cannot answer
- Bibliography for further study
Imagine you were hired to make a detailed map of New York’s Central Park. You spend years exploring and documenting every walkway, field, pond, bridge, garden and paddock. Then one day, you discover a door in the ground. Opening this door, you find a staircase, and as you descend the stairs, you are amazed to see… another city; even bigger than New York. It’s streets stretch far and wide, connecting all the towns and cities in New Jersey, Connecticut and beyond
Such an event occurred in the mid 1990’s as biologists found new receptors on cells throughout the body that appeared to be connected to the brain, autonomic and sympathetic nervous systems, the gut, endocrine and the immune systems. Ultimately, they identified signaling molecules that functioned within this new system, and because these molecules activated known cannabinoid receptors, they called this vast network the endocannabinoid system or ECS.
As you can see from the graph below, this led to an explosion of research around the world. As of this writing, more than 4,000 English language studies have been published, exploring everything from plant genetics (eg developing strains with higher levels of CBD) to processing technologies and of course health and wellness benefits.
Number of published studies on cannabidiol (CBD)
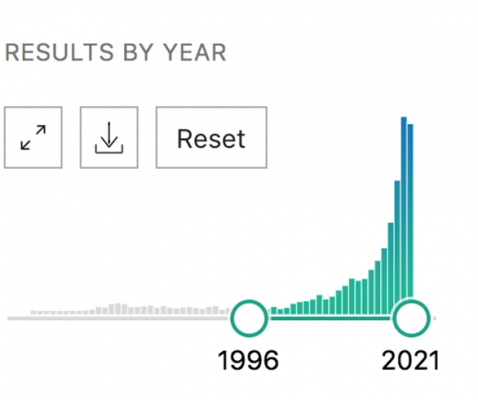
Q: With so much research, why is the CBD arena so confusing?
A: I can think of three major factors.
- We are still in the early stages of CBD research. Because the ECS affects so many biological functions, anything that alters this activity needs a great deal of research in multiple areas of science. Fortunately, this starts with safety, and the safety profile of CBD is now well established. There are, of course, important cautions for people with serious medical conditions or those taking prescribed medications. It’s always important to consult with your health professional before using any natural health product.
- Poor quality and unscrupulous marketers. Because CBD is derived from hemp, dozens of companies sprang up selling “CBD-rich hemp oil.” That could be a bottle of hemp oil normally used for cooking, with a few drops of CBD added. As you can imagine, people who purchased those products experienced no benefit. Fortunately, leading manufacturers started to label products with the CBD content specified in milligrams, and supported by independent lab analysis.
- Even with quality products, the effective dose range is extremely wide. Ready for a little ECS Education? CBD is one of a group of compounds known as cannabinoids. Our bodies also make cannabinoids, mostly from essential fats like EPA and DHA. These are called endocannabinoids; (“endo” means “within,” as in within the body) and the amount of endocannabinoids that we make varies, depending on many factors including diet, age, stress level, exercise, and other lifestyle factors. People who supplement with fish oil (a great source of EPA and DHA) may need a lower dose of CBD to experience benefits, whereas another person with low levels of EPA and DHA might need a much higher dose. What’s more, even with an ideal diet, endocannabinoid synthesis declines with advancing age. Bottom line, many (perhaps most) people are under-dosing, and at this time, the only way to know is to experiment. The good news is that studies have been conducted with doses as high as 1,000 mg/day with no serious adverse effects.
One recent study may shed some light on the dosing issue. Researchers trying to measure the anxiety-reducing effect of CBD presented 57 healthy subjects with a stressful task: giving a speech in front of a live audience. Prior to the public speaking challenge, 15 subjects were given 150 mg of CBD, 15 received 300 mg, 12 received 600 mg and 12 received a look-alike placebo. The investigators measured blood pressure and heart rate, and each participant rated their subjective feelings of anxiety. The results were reported as “Compared to placebo, pretreatment with 300 mg of CBD significantly reduced anxiety during the speech. No significant differences were observed between groups receiving CBD 150 mg, 600 mg and placebo.”
REF: REF: Braz J Psychiatry. 2019 Jan-Feb; 41(1): 9–14.
Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Ila M. Linares, et al.
In other words, these researchers identified a bell-shaped curve of benefits. 150 mg of CBD didn’t have much of an effect, nor did 600 mg. But a dose of 300 mg produced a significant reduction in anxiety. Similar dose-benefit curves have been identified in studies looking at inflammation and chronic pain. This would not be practical using a conventional CBD oil standardized to 500 mg per oz. Fortunately, a new processing technology has been developed that achieves potencies SIX TIMES higher than conventional tinctures.
The NANO difference. Imagine a 1 oz dropper bottle guaranteed to provide 3,000 mg of CBD. A starting dose would be 9 or 10 drops. Now imagine that the potency is only half the story, because when you’ve reduced the particle size to under 100 nanometers, the material becomes water-dispersible. This means dramatically better bioavailability compared to an oil. Think enhanced relaxation, rest, recovery, and well-being. Yes, the bloodstream can carry fats and oils, but only attached to carrier molecules, whereas water-soluble compounds have much greater distribution through the body, thus supporting the ECS and associated benefits.
Q: Is Nano CBD going to cost 6 x as much as a conventional tincture?
A: Looking at the market today, high-potency products are selling in the range of $125 to $175 per oz. Altea Health Sciences NANO CBD is $89, member price: $75, with discounts on two-packs. For most people, a bottle will last 2 or 3 months, so there is a significant cost savings relative to conventional tinctures, gummies or gelcaps.
Three factors allow us to offer these prices.
- Plant genetics. We start with a proprietary strain of hemp with the highest concentration of CBD.
- NANO processing extracts the maximum amount of CBD and related cannabinoids.
- We are not MLM
Guaranteed quality
Every batch of Altea NANO CBD is tested by independent lab assays for purity and potency.
There are some questions we cannot answer
To comply with Food and Drug Administration (FDA) regulations, we cannot make disease claims or speak directly about the treatment or prevention of any disease. FDA regulations are very clear on this. Making disease claims for a dietary supplement automatically places that product in the category of an unapproved drug. That violation carries severe penalties, so we forgo direct communication regarding specific diseases, for the good of each and every person taking Altea Health Sciences products.
Regardless of the amount of research-backed studies around cannabinoids, or the fact that Natalie and I have six decades of clinical experience, the FDA could revoke our ability to sell CBD if they felt we were giving instructions for treating specific diseases.
Saying that, we are happy to refer you to trusted scientific resources like pub med https://pubmed.ncbi.nlm.nih.gov/ and reliable educational organizations like the Realm of Caring Foundation: https://realmofcaring.org/
Moving forward: What you can do
- We believe that knowledge is power, and there are many ways to network. Our customers and Healthy Skeptics Members can respond to each other in comments on social media. While we’re not allowed to endorse any testimonials (more FDA restrictions), you can share your personal experience with others, or ask other Healthy Skeptics users how they incorporate our products into their health routines.
- Subscribe to our newsletter and share it with your friends and family.
- Write or email your congressional representatives and tell them how CBD products have contributed to your health. Remember, these men and women are the only thing standing in the way of a wholesale takeover of the CBD industry by Big Pharma. Let them know that you want to have OTC access to these natural compounds, and that you are smart enough to select quality products from leading suppliers.
- Educate yourself. You don’t need an advanced degree in biology to understand a lot of the biomedical literature. Take a good look at this chart. Discuss it with your health care professional, so that we can all gain the most from this extraordinary discovery.
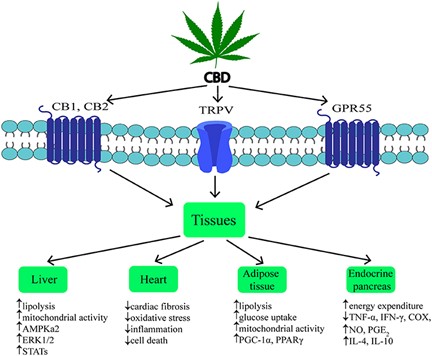
Quote from the above research summary:
“CBD is considered as a potential therapeutic agent due to its anti-inflammatory, antioxidant, anti-tumor, neuroprotective, and potential anti-obesity properties.”
REF:
Frontiers in Endocrinology, 04 March 2020 | https://doi.org/10.3389/fendo.2020.00114
Phytocannabinoids: Useful Drugs for the Treatment of Obesity? Special Focus on Cannabidiol. Patrycja Bielawiec, Ewa Harasim-Symbor and Adrian Chabowsk
Bibliography for further study
- Cannabis and Cannabinoid Research Vol. 2, No. 1.
An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Kerstin Iffl and and Franjo Grotenhermen
Published Online:1 Jun 2017https://doi.org/10.1089/can.2016.0034
- Free Radic Biol Med. 2011 Sep 1;51(5):1054-61. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation and oxidative stress. Booz GW.
Abstract
This review discusses recent studies suggesting that cannabidiol may have utility in treating a number of human diseases and disorders now known to involve activation of the immune system and associated oxidative stress, as a contributor to their etiology and progression. These include rheumatoid arthritis, types 1 and 2 diabetes, atherosclerosis, Alzheimer disease, hypertension, the metabolic syndrome, ischemia-reperfusion injury, depression, and neuropathic pain.
- Biochem Pharmacol. 2014 Feb 1;87(3):489-501. Increase of mesenchymal stem cell migration by cannabidiol via activation of p42/44 MAPK. Schmuhl E, Ramer R, Salamon A, Peters K, Hinz B.
Abstract
Migration and differentiation of mesenchymal stem cells (MSCs) are known to be involved in various regenerative processes such as bone healing. However, little is known about the pharmacotherapeutical options aiming at the mobilization and differentiation of MSCs. The present study therefore focused on cannabinoids which have been demonstrated to exhibit tissue healing properties. Using Boyden chamber assays, the non-psychoactive phytocannabinoid cannabidiol (CBD) was found to increase the migration of adipose-derived MSCs in a time- and concentration-dependent manner. Moreover, the promigratory effect of CBD was antagonized by inhibition of the p42/44 mitogen-activated protein kinase (MAPK) pathway which became activated upon CBD treatment. Additional evidence for a functional effect of CBD on MSCs was provided by experiments demonstrating long-term stimulation with CBD to induce differentiation of MSCs into the osteoblastic lineage as evidenced by increased mineralization assessed by cresolphthalein complexone assay and enhanced activity of alkaline phosphatase. Collectively, this study demonstrates CBD to promote the migration of MSCs via activation of the CB₂ receptor and inhibition of GPR55 and to induce osteoblastic differentiation. CBD may therefore recruit MSCs to sites of calcifying tissue regeneration and subsequently support bone regeneration via an osteoanabolic action on MSCs.
- J. Cell. Biochem. 118: 1531–1546, 2017. Cannabidiol Activates Neuronal Precursor Genes in Human Gingival Mesenchymal Stromal Cells. Thangavelu Soundara Rajan, et al.
ABSTRACT
In the last years, mesenchymal stromal cells (MSCs) from oral tissues have received considerable interest in regenerative medicine since they can be obtained with minimal invasive procedure and exhibit immunomodulatory properties. This study was aimed to investigate whether in vitro pre-treatment of MSCs obtained from human gingiva with Cannabidiol (CBD), may promote human gingiva derived MSCs to differentiate toward neuronal precursor cells. Specifically, we have treated the hGMSCs with CBD (5 µM) for 24 h in order to evaluate the expression of genes involved in cannabidiol signaling, cell proliferation, self-renewal and multipotency, and neural progenitor cells differentiation. Next generation sequencing (NGS) demonstrated that CBD activates genes associated with G protein coupled receptor signaling in hGMSCs. Genes involved in DNA replication, cell cycle, proliferation, and apoptosis were regulated. Moreover, genes associated with the biological process of neuronal progenitor cells (NCPs) proliferation, neuron differentiation, neurogenesis, and nervous system development were significantly modulated. From our results, we hypothesize that human gingiva-derived MSCs conditioned with CBD represent a valid method for improving the hGMSCs phenotype and thus might be a potential therapeutic tool in the treatment of neurodegenerative diseases.
- I’ll just leave this right here:
Daily use of cannabidiol (‘CBD’) oil may be linked to lung cancer regression
Here’s the published report:
https://casereports.bmj.com/content/14/10/e244195
- Clinical Trial Postgrad Med. 2020 Jan;132(1):56-61. doi: 10.1080/00325481.2019.1685298.
Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: a prospective cohort study. Alex Capano , Richard Weaver, Elisa Burkman
Abstract
Context: Chronic pain is highly prevalent in most of the industrialized nations around the world. Despite the documented adverse effects, opioids are widely used for pain management. Cannabinoids, and specifically Cannabidiol, is proposed as an opioid alternative, having comparable efficacy with better safety profile. Objectives: We aim to investigate the impact of full hemp extract cannabidiol (CBD) on opioid use and quality of life indicators among chronic pain patients. Methods: An initial sample of 131 patients was recruited from a private pain management center’s investigative population. Ninety-seven patients completed the 8-week study. The primary inclusion criteria included patients between 30 and 65 years old with chronic pain who have been on opioids for at least 1 year. Data were collected at three different time points: baseline, 4, and 8 weeks. Opioid and other medication use were evaluated via the medication and psychiatric treatment receipt. Improvement was evaluated using four indices: Pain Disability Index (PDI-4); Pittsburgh Sleep Quality Index (PSQI), Pain Intensity and Interference (PEG); and Patient Health Questionnaire (PHQ-4). Results: Over half of chronic pain patients (53%) reduced or eliminated their opioids within 8 weeks after adding CBD-rich hemp extract to their regimens. Almost all CBD users (94%) reported quality of life improvements. The results indicated a significant relationship between CBD and PSQI (p = 0.003), and PEG (p = 0.006). There was a trend toward improvement but no significant relationship between CBD use and PHQ and PDI. Conclusion: CBD could significantly reduce opioid use and improve chronic pain and sleep quality among patients who are currently using opioids for pain management. Key Message: This is a prospective, single-arm cohort study for the potential role of cannabinoids as an alternative for opioids. The results indicate that using the CBD-rich extract enabled our patients to reduce or eliminate opioids with significant improvement in their quality of life indices.
Download Complete Newsletter